The research group led by Jun-Long Zhang from College of Chemistry and Molecular Engineering, Peking University published a research paper entitled "Gallium Triggers Ferroptosis through a Synergistic Mechanism" in the Angew. Chem. Int. Ed., which found that gallium interferes with cell redox homeostasis and induces ferroptosis through a synergistic mechanism (Angelw. Chem. Int. Ed., 2023, 62, e202307838).
In the history of metallodrug, gallium is the metal second only to platinum in anti-tumor research. Gallium compounds are widely used clinically in the treatment of antibacterial, anti-inflammatory, anti-tumor, and bone-metabolic diseases. In terms of anti-cancer mechanisms, due to the high similarity in charge and ion radius properties between gallium ions (Ga3+) and iron ions (Fe3+), trivalent gallium is considered as a redox inert "iron surrogate" that can interfere with the homeostasis of Fe3+ in organisms. Additionally, it serves as a competitive inhibitor of Fe3+, disrupting the activity of iron-containing enzymes (such as ribonucleotide reductase) and leading to cell death. For a long time, the "iron surrogate" theory has been believed as the basic theory of gallium’s modes of action (Eur. J. Inorg. Chem., 2022, e202100953). In recent years, cases of gallium complex induced ferroptosis have been reported, but further research on the role of gallium in ferroptosis still requires further clarification.
Zhang Jun-Long's research group and collaborators previously reported the first case of a Ga(III)-salen complex (Ga-1) that can target protein disulfide isomerase (PDI) (Angew. Chem. Int. Ed., 2020, 59, 20147) causing programmed cell death induced by endoplasmic reticulum stress, achieving tumor treatment. Recently, they found that the strong Lewis acidity of Ga3+ enables Ga-1 to bind to negatively charged phospholipids and hydroxyl groups on the mitochondrial membrane through coordination interaction, altering the proton gradient throughout the membrane. As a consequence, iron-containing cofactors will be released from the mitochondrial inner membrane. Furthermore, Ga-1 induces an increase in the expression of stress protein heme oxidase 1 (HMOX-1), resulting in mitochondrial iron overload and inducing cellular lipid peroxidation. On the other hand, they found that Ga-1 can disrupt the glutathione system by inhibiting PDI, inducing downregulation of SLC7A11 (responsible for cysteine uptake) and GPX4 (responsible for lipid peroxide clearance in the glutathione system), thereby disrupting cellular antioxidant capacity. This synergistic mechanism makes the anti-tumor effect more significant. Unlike the traditional "iron surrogate" theory, this study offers important clues for elucidating the mode of action of Ga-1 and provides new perspectives for the molecular design of novel gallium anticancer drugs.
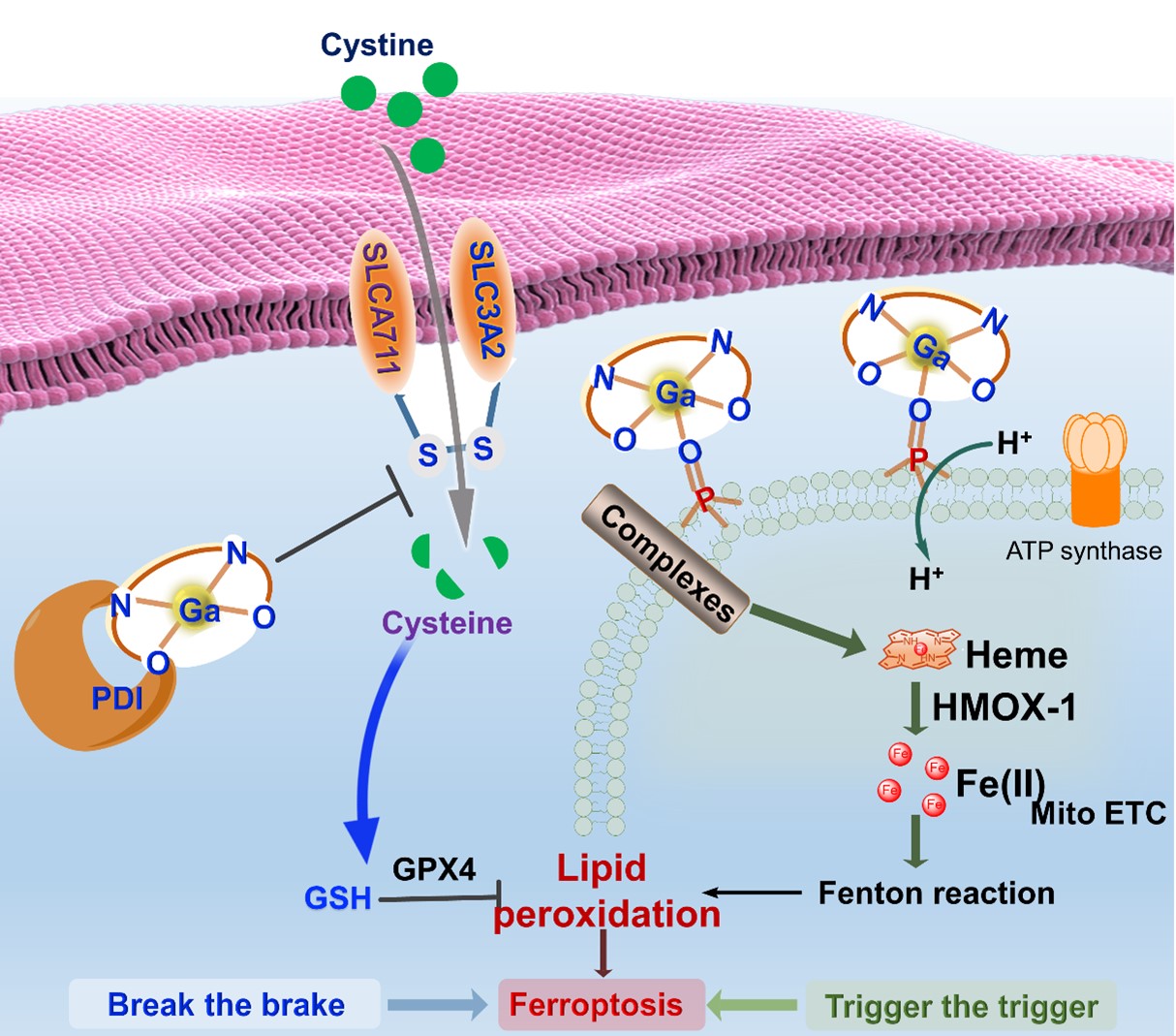
Fig. 1 Proposed synergistic mechanism of ferroptosis induced by Ga-1: “trigger the trigger, break the brake”. Ga-1 induces ETC dysfunction by binding to phospholipids on mitochondrial membrane, thus promoting iron release to trigger lipid peroxidation. Ga-1 also disrupts the antioxidant GSH system, through PDI inhibition.
Professor Jun-Long Zhang is the corresponding author of the paper, and Xin-Xin Peng, a PhD student from College of Chemistry and Molecular Engineering, Peking University, is the first author. This work has been supported by the National Natural Science Foundation of China, the Chemistry and Chemical Engineering Guangdong Laboratory, and the Beijing National Laboratory for Molecular Sciences in China.
Original link for the paper: https://onlinelibrary.wiley.com/doi/10.1002/anie.202307838