The team led by Prof. Bingjun Xu at the College of Chemistry and Molecular Engineering, Peking University, recently demonstrated the impact of site heterogeneity of Cu on the electrochemical CO2 reduction reaction. The research results were published in Nature Catalysis, entitled “Experimental Evidence of Distinct Sites for CO2-to-CO and CO Conversion on Cu in the Electrochemical CO2 Reduction Reaction”.
Electrochemical CO2 reduction reaction (CO2RR) has been widely acknowledged as a promising strategy to close the carbon circle by converting CO2 into valuable C2+ hydrocarbons and oxygenates powered by renewable electricity. Cu is the only metal capable of selectively reducing CO2 to C2+ products with decent yields. Despite intense research efforts, two key points with impactions for catalyst design remain unresolved, i.e., whether the CO2-to-COc onversion and the further conversion of CO are independent steps; and whether the two reactions occur on the same sites on Cu.
To address these mechanistic questions, co-electrolysis of mixtures of CO2 and CO with varying compositions was conducted on a commercial Cu catalyst to determine the effect of feed composition on the rate and product distribution. Co-electrolysis of CO2/CO mixtures exhibited higher rates than the electrolysis of either molecule (CORR or CO2RR, Fig. 1a). Combining with in situ spectroscopic and isotopic labeling investigations (Figs. 1b and 2a,b), they demonstrated that the presence of CO2 enhanced the Cu-catalyzed CORR to multicarbon products without providing an additional source of carbon.
Analysis of isotopologue distributions of products in the co-electrolysis of mixtures of 13CO/12CO2 showed significant deviations from those expected of the binomial distribution (Fig. 2c). Combined with the relative surface coverages of 12CO and 13CO determined spectroscopically (Fig. 2d), we suggested there were at least two distincttypes of sites on Den Cu, and the migration of adsorbed CO among different types of sites was slow on the reaction time scale.
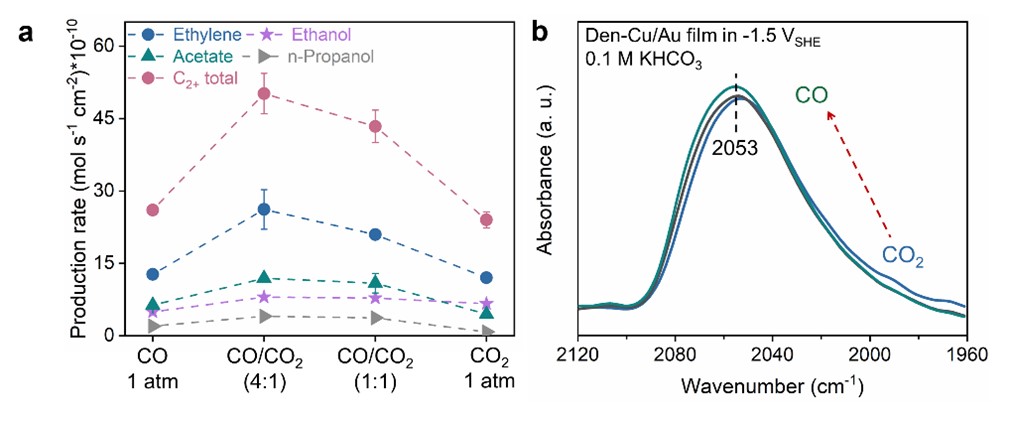
Fig. 1 (a) Product formation rate of total C2+ in the co-electrolysis of CO/CO2 mixtures with varying compositions. (b) In situ SEIRA spectra of adsorbed CO at similar conditions with the atmosphere gradually switching from CO2 (blue trace) to CO (green trace).
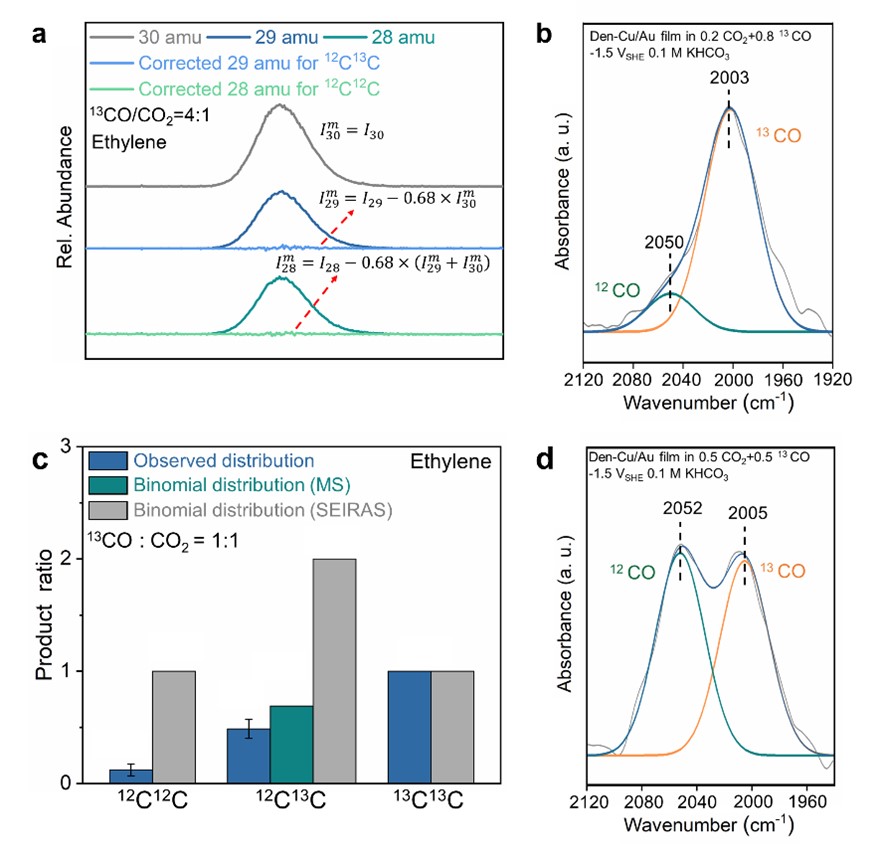
Fig. 2 (a) Distribution of isotopologues of ethylene produced in the co-electrolysis of 13CO/12CO2 (0.8 atm/0.2 atm). (c)Observed distribution (blue bars) and expected binomial distributions of isotopologues of ethylene produced in the co-electrolysis of 13CO/12CO2 (0.5 atm/0.5 atm). (b) In situ SEIRA spectra of adsorbed CO in (b) 13CO/12CO2 (0.8 atm/0.2 atm) and (d)13CO/12CO2 (0.5 atm/0.5 atm).
Based on these observations, they proposed a two-site model, in which one type of site was more efficient for the CO2-to-CO conversion (CuCO2) and the other favored the further reduction of CO to C2+ products (CuCO), which was able to rationalize the observed reactivity trend (Fig. 3a). Analysis based on a proposed two-site model shows that CO adsorbed on CuCO is at least six times more active towards the formation of C2+ products compared to that on CuCO2. Co-electrolysis experiments conducted on single crystal Cu facets indicate that CuCO2 corresponds to Cu(111)-like sites and CuCO is associated with undercoordinated Cu sites (Fig. 3b,c).
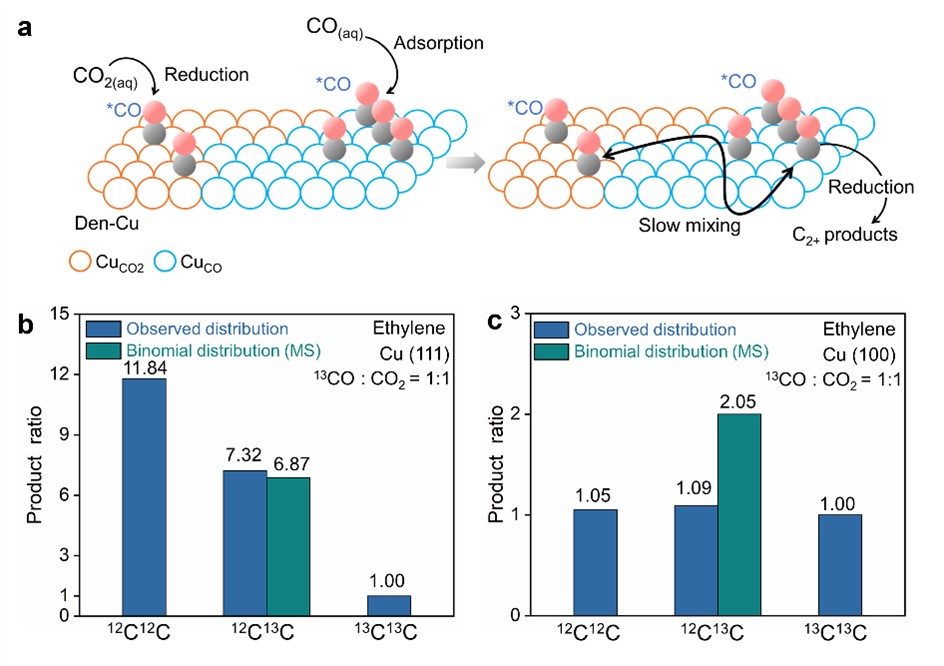
Fig.3 (a) Schematic the proposed two-site model on Cu. Distribution of ethylene isotopologues produced in the co-electrolysis of 13CO/12CO2 (0.5 atm/0.5 atm) on (b) Cu(111) and (c) Cu(100).
Prof. Bingjun Xu, a faculty at the College of Chemistry and Molecular Engineering, Peking University, is the corresponding author.Dr. Wenqiang Gao, a postdoctoral researcher at the College of Chemistry and Molecular Engineering of Peking University, and Yifei Xu, a Ph.D. candidate at the College of Chemistry and Molecular Engineering of Peking University, are the joint first authors of this paper. Collaborators including Linke Fu and Dr. Xiaoxia Chang contributed to this project.This research was jointly supported by the Beijing National Laboratory for Molecular Sciences and the China Postdoctoral Science Foundation.
Original link for the paper: https://www.nature.com/articles/s41929-023-01002-6